Malignant central nervous system (CNS) tumors are dubbed “the worst cancer” by Scientific American (Stetka, 2019). Gliomas are CNS tumors originating from glial cells (non-neuronal cells that provide support to neurons) that comprise 35% of all CNS cancers (Hauser, 2021). Gliomas are separated into two categories and four grades: low-grade (1-2) gliomas (pLGGs) and high-grade (3-4) gliomas (pHGGs) (Hauser, 2021). This classification considers growth patterns, tumor behavior, and genetic driver mutations to group CNS tumors (Hauser, 2021). In general, a higher grade represents a more aggressive tumor (Louis et al., 2021). While pLGGs have an overall survival rate of over 90%, pHGGs have a survival rate of less than 10% (Hauser, 2021). Glioblastomas (GBMs), first classified in 1926 by Percival Bailey and Harvey Cushing (Stoyanov et al, 2018), are a specific type of grade four pHGG that is notorious for being extremely common, comprising 57% of all gliomas (Tan et al., 2020), yet extremely deadly, with only 5.8% of patients surviving five or more years following diagnosis (Tan et al., 2020). It is known that glioblastomas have a poor overall prognosis due to the existence of several subtypes that require different treatments, as well as rapid tumor growth (Tamimi et al., 2017). Because of this cancer’s extraordinary prevalence, aggressive nature, and complicated treatment processes, neuroblastomas present a terrifying case of cancer that has seemingly no path to a cure.
Like all gliomas, glioblastomas are mostly idiopathic and are caused by a genetic mutation in neural glial cells that causes rapid, uncontrolled cell division (Stetka, 2019). This creates a tumor, a mass of invasive cells that infiltrates healthy areas and consumes large amounts of energy (Stetka, 2019). Glioblastomas are a relatively common malignant primary brain tumor among all populations, with an average age-adjusted incidence rate of 3.19 per 100,000 persons in the United States (Tamimi et al., 2017). The median age at diagnosis is 65, and the 75-84 age group experiences the highest rates of disease (Tan et al., 2020). There is a higher rate of occurrence (1.6 times higher) in males than females, and a higher rate of occurrence (2 times higher) in Caucasians compared to Africans (Tamimi et al., 2017). Asians and American Indians have a lower incidence (up to 40% lower) than all other groups (Tamimi et al., 2017). Some data suggest that there is a higher rate of occurrence in highly developed, industrialized populations as well (Ohgaki et al., 2005). Glioblastomas almost always occur in the cerebrum (supratentorial region – frontal, temporal, parietal, and occipital lobes of the brain) (Tamimi et al., 2017). Research shows that a strong risk factor for glioblastomas is exposure to ionizing radiation (Tan, et al., 2020). There is an inverse association between the occurrence of glioblastomas and the presence of allergies (Tan, et al., 2020). Rare genetic syndromes like Li-Fraumeni syndrome and Lynch syndrome cause this cancer, but these account for less than one percent of all cases (Tan, et al., 2020). Interestingly, the incidence of glioblastomas is on the rise in many countries, while survival rates remain low (Grech et al, 2020). This rise in incidence could be attributed to an aging population, overdiagnosis, inhalation of pollutants containing carcinogens (substances that trigger mutations and cause cancer), or other currently unexplained reasons (Grech et al., 2020).
The effects and progression of glioblastoma may be even scarier than its prevalence. Despite advancements in multimodal treatment options, glioblastoma remains an incurable disease (Fritz et al., 2016). Over the course of the disease, patients can experience a vast variety of symptoms, including increased intracranial pressure, headaches, incontinence, cognitive dysfunction, and progressively worsening neurologic deficits (Fritz et al., 2016). Seizures are an early symptom in up to 25% of all patients and can occur as a later symptom in up to 50% of patients (Davis, 2016). This combination of factors leaves a median survival time of 15 months for glioblastoma patients (Fritz et al., 2016), a dire number showing the horrific capabilities of the disease. The elderly have a statistically increased susceptibility to glioblastomas, and with higher incidences of multiple other conditions at an advanced age, successful treatment is rare, with patients normally succumbing to either the glioblastoma or other factors (Tan et al., 2020). Often, the families of the patients are devastated by the long-term implications of a glioblastoma diagnosis (Tan et al., 2020).
Due to the nature of glioblastoma progression, treatment options are more restricted, and prognosis is generally poor (Tan et al., 2020). As with other CNS tumors, the standard approach to glioblastomas is maximal safe surgical resection to reduce tumor size (Tan et al., 2020). In most cases, this pertains to gross total resection, the complete removal of a tumor, and surrounding damaged tissue (Tan et al, 2020). For glioblastomas specifically, surgery is followed by radiotherapy (high-energy X-rays used to destroy cancer cells) and chemotherapy in the form of temozolomide, an anti-cancer drug (Tan et al, 2020). Patients presenting with seizures are also given antiepileptic drugs (Davis, 2016). In the elderly, temozolomide is often used without radiotherapy due to the risks of intense radiation (Tan, et al, 2020). However, in the event of recurrent tumors or a late diagnosis, no treatment plan is defined (Tan et al, 2020). In these cases, death is often inevitable and the sole treatment option is supportive care and end-of-life treatment, aiming to ease suffering and provide closure in the last 3 months of life (Fritz et al, 2016). Patient decision-making is a controversial topic for glioblastoma care due to the inability of most patients to make decisions on their treatment plans due to progressive loss of brain function (Fritz et al., 2016). Thus, treatment is extremely difficult because of the cancer’s aggressiveness and small effective treatment window.
Overall, the combination of high rates of occurrence, aggressive symptoms, and lack of concrete treatment options creates a daunting scenario in which the developmental progress of an effective treatment for glioblastomas cannot keep up with its painstaking death toll. It is critical that humanity works together and allocates its resources to the continued development of a cure for this terrible disease and cancer in general to save countless lives.
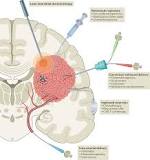
Figure 1. Glioblastoma tumor (red) triggering growth of new blood vessels (pink) and invading nearby neurons (blue).
 Improvements: Talk about metastasis (or lack thereof), specific gene mutations, classification of glioblastomas and differences in treatment, and famous deaths
Improvements: Talk about metastasis (or lack thereof), specific gene mutations, classification of glioblastomas and differences in treatment, and famous deaths
References
Davis, M. (2016). Glioblastoma: Overview of disease and treatment. Clinical Journal of Oncology Nursing, 20(5), S2-S8. https://doi.org/10.1188/16.cjon.s1.2-8
Fritz, L., Dirven, L., Reijneveld, J., Koekkoek, J., Stiggelbout, A., Pasman, H., & Taphoorn, M. (2016). Advance care planning in glioblastoma patients. Cancers, 8(11), 102. https://doi.org/10.3390/cancers8110102
Grech, N., Dalli, T., Mizzi, S., Meilak, L., Calleja, N., & Zrinzo, A. (2020). Rising incidence of glioblastoma multiforme in a well-defined population. Cureus. https://doi.org/10.7759/cureus.8195
Hauser, P. (2021). Classification and treatment of pediatric gliomas in the molecular era. Children, 8(9), 739. https://doi.org/10.3390/children8090739
Louis, D. N., Perry, A., Wesseling, P., Brat, D. J., Cree, I. A., Figarella-branger, D., Hawkins, C., Ng, H. K., Pfister, S. M., Reifenberger, G., Soffietti, R., Von deimling, A., & Ellison, D. W. (2021). The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncology, 23(8), 1231-1251. https://doi.org/10.1093/neuonc/noab106
Ohgaki, H., & Kleihues, P. (2005). Epidemiology and etiology of gliomas. Acta Neuropathologica, 109(1), 93-108. https://doi.org/10.1007/s00401-005-0991-y
Stetka, B. (2019, March 27). New strategies take on the worst cancer–Glioblastoma. Scientific American. Retrieved July 28, 2023, from https://www.scientificamerican.com/article/new-strategies-take-on-the-worst-cancer-glioblastoma/
Stoyanov, G. S., & Dzhenkov, D. L. (2018). On the concepts and history of glioblastoma multiforme – morphology, genetics and epigenetics. Folia Medica, 60(1), 48-66. https://doi.org/10.1515/folmed-2017-0069
Tamimi, A. F., & Juweid, M. (2017). Epidemiology and outcome of glioblastoma. Glioblastoma, 143-153. https://doi.org/10.15586/codon.glioblastoma.2017.ch8
Tan, A. C., Ashley, D. M., López, G. Y., Malinzak, M., Friedman, H. S., & Khasraw, M. (2020). Management of glioblastoma: State of the art and future directions. CA: A Cancer Journal for Clinicians, 70(4), 299-312. https://doi.org/10.3322/caac.21613